HQMS - ABOUT THE SYSTEM
- We have a quality management system (HQMS) in place to conduct the pharmacovigilance activities that have been implemented to meet pharmacovigilance legislation and guidelines.
- Our Quality system keeps all the information up to date and readily accessible and thus simplifies the process, save time and money, and provide quality assurance for our clients.
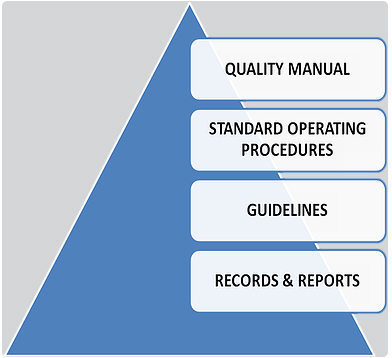
LEVEL I: Quality Manual, which includes the Quality Policy.
LEVEL II: Standard Operating Procedures, which define the detailed operational requirements of the Quality System within the company.
LEVEL III: Quality system procedural guidelines that support standard operating procedures.
LEVEL IV: The fourth level represents records and reports as documented evidence of compliance to established written procedures.
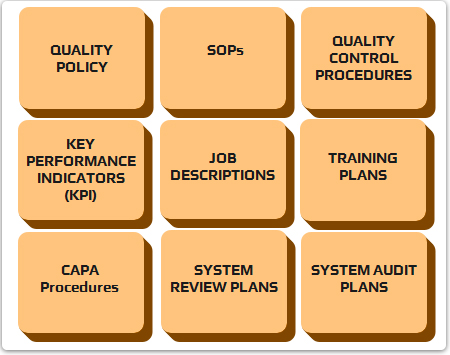
HQMS - ESSENTIAL FEATURES
- Extensible high performance Quality system with advanced technology
- Sophisticated Quality department
- Documented procedures and work instruction documents
- Quality reviews at each step of ICSR processing with detailed guidelines
- Well established Performance Metrics for identification and monitoring of key performance indicators to help measure performance and effectiveness.
- Authorization based system
- Integrated Quality trackers
- Action based Quality system
- Record retentions
- Electronic Signatures
HQMS - THE PROCESS
1. QUALITY PLANNING
Clear Written Standard Operating Procedures and Quality trackers
2. QUALITY CONTROL
Quality Checks at every stage (Data collection; Data management; Data processing etc.) of case documentation is verified for compliance, quality and integrity of data.
3. QUALITY ASSURANCE
Continuous monitoring and evaluation of Quality system to make sure how effectively the structures and processes have been established and how effectively the processes are being carried out.
4. QUALITY IMPROVEMENTS
Correcting and improvement in the structures and processes and the carrying out of those processes as necessary
Contact us for help, additional info or assistance and we'll be with you in no time!